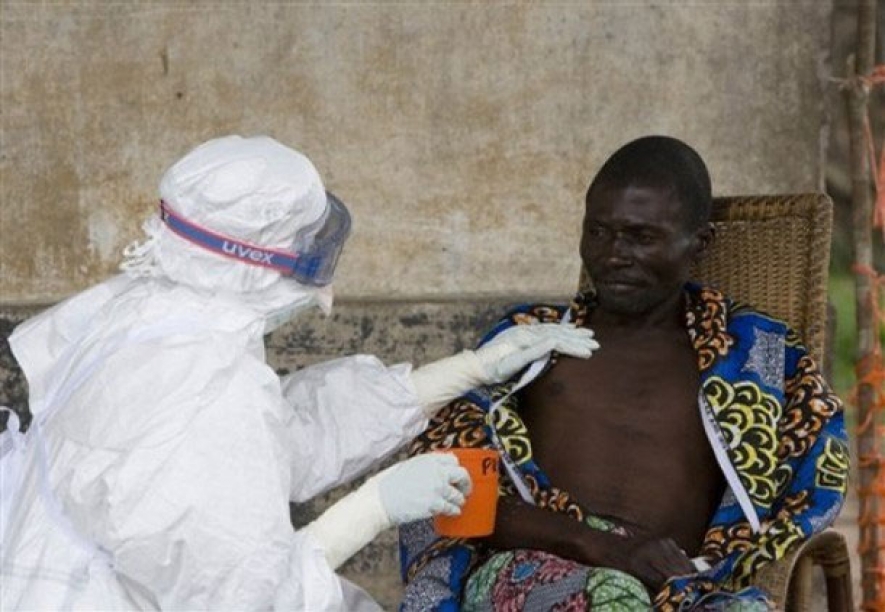
Still, the agency warned it's not clear whether any of these will work against the deadly virus that has already killed at least 4,877 people this year in West Africa.
Dr. Marie-Paule Kieny from the U.N. health agency told reporters that those doses could be available in 2015 if early tests proved that the two leading experimental vaccines are safe and provoke enough of an immune response to protect people from being infected with Ebola.
Trials of those two most advanced vaccines —one developed by GlaxoSmithKline in cooperation with the U.S. National Institutes of Health, the other developed by the Canadian Public Health Agency — have already begun in the U.S., U.K. and Mali.
"The vaccine is not the magic bullet. But when ready, they may be a good part of the effort to turn the tide of this epidemic," Kieny said.
If early data from the ongoing tests are promising, larger trials in West Africa would offer the shot to health workers and others at high risk of catching Ebola as soon as December, Kieny said. Previously those trials weren't starting until January.
GSK said it might be able to make about 1 million doses of their vaccine per month by the end of 2015, assuming that some logistical and regulatory hurdles can be overcome.
"The message we heard from WHO that the people fighting the epidemic will be among the first to test Ebola vaccines and treatments is exactly the one we needed to hear," Dr. Bertrand Draguez, medical director for Doctors Without Borders, said in a statement. "This needs to be followed by a massive roll-out of vaccines to the general population once their efficacy is proven."
Kieny also said five other possible Ebola vaccines should start being tested in March, but she gave no details about who is making them or where those five vaccines would be tested.
She said details about getting the vaccines to West Africa had yet to be worked out, including who would pay for immunization campaigns — which weren't planned to start before June at the earliest. Kieny said the charity Doctors Without Borders pledged to create a vaccine fund and other organizations, including the World Bank, might help buy the vaccines.
She also acknowledged that, given the speed at which these experimental vaccines are being rolled out, "there will certainly not be as much known in terms of their safety as would be normal." Kieny said Britain had proposed creating a fund that would offload liability from pharmaceutical companies in case any bad side effects emerge from the shots.
In Brussels on Friday, the European Union and its 28 member nations managed to create a 1 billion-euro ($1.26 billion) fund to fight the Ebola outbreak. Britain's contribution of 205 million pounds ($329 million) was the largest among the EU nations.
"Helping West Africa to cope with the crisis is the most effective way to prevent a serious outbreak of the disease elsewhere," the EU leaders said at the end of a two-day summit. "The scale of the epidemic is a threat not only to the economy and the stability of the affected countries, but also to the region as a whole. "
In Beijing, China's president pledged to provide $81 million in aid to help fight Ebola.(KH)